-


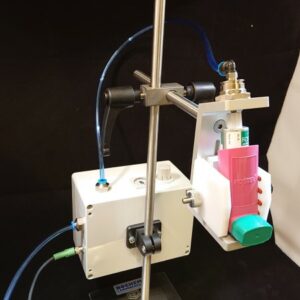
TrB III Trigger Box – All-in-One Flow Controller
The TrB III Trigger Box ensures compliance with standard pharmacopeial methods, both recording and storing key system parameters, including the actual flow rate and run duration. Many inhaler test methods rely on critical flow conditions across the flow control valve, aiming to ensure the same flow rate on each test. But the TrB III does more – actually measures the flow of each test – so, there are no assumptions.
A calibrated laminar flow element (LFE) internal to each TrB III enables the user to set the flow rate at the beginning of a test sequence; with this LFE, the TrB III then records the flow rate of each test, ensuring against drift, leaks, and other non-ideal behavior that may introduce variability in test results. The TrB III also records the other more traditional run-time parameters, such as the test duration, the pressure drop across the inhaler device (P1), and the flow control pressure ratio (P3/P2, critical flow if ≤ 0.5).1
Additional user-friendly functions are leak checking and synchronized device actuation by using the integrated output port. Device actuation enables the flow to start simultaneously with dose actuation of a metered-dose inhaler, allowing a user- defined, fixed flow volume for MDI total dose testing.
Download AB Fia folder Trigger Box TrB III.
Key features are:
- Flow actuation: 0-60 min, 0.1s resolution
- Actuation counter: Resettable 0-999
- Foot switch: Yes
- Display: 7” touch
- P1 measurement: Yes, 0-16 kPa
- P3/P2 measurement: Yes
- Flow measurement: Calibrated 0 – 120 l/min (operating range possibly higher)
- Automatic leak test: Yes
- Printable data: Prints new actuations continuously or print all actuations from reset.
- Date/time of first dose
- Instrument ID
- Instrument ver
- Flow ”on” time
- External relay timing
- Atmospheric pressure
- Dose number
- P1 and flow
- P3/P2 (if < 0.5)
- Relay output for actuation of external equipment: Yes, configurable timing of output relative vacuum opening.
- Displayed history of recent actuation data: All actuations from reset.
- Interfaces:
- Relay output for actuation of external equipment
- Foot switch actuator
- USB for CSV export
- Dimensions (cm): 34x13x13
Download the pdf TrB III – All in one Flow Controller
-


Glass Frit Impinger DUSA
Used as an alternative to the DUSA after proper equivalence testing (ask us how!). Used by many of the leading companies within OINDP testing. This filter comes in various forms but adheres to the principles which was published in a USP stimuli article 1993. 1 It lends itself to high-capacity testing and automation since it is washable with good recovery and minor carry-over.
- 1. Hugosson, S., J. Lindberg, T. Lööf, B. Olsson, ”Proposals for Standardized Testing of Powder Preparations for Inhalation”, Pharm Forum, Vol 19-3, 5458-66, 1993
-

Dose Station Turbuhaler ®
This is our dedicated delivered dose uniformity test station for Turbuhaler ®, using the brilliant impinger filter dose collector. The latter is washable in-situ and can be ready for the next dose as part of the wet chemistry work-up procedure. The user works with the equipment in a fixed process which starts by the user placing the device in an inlet which mates with the dose collector. The inlet is a highly sophisticated construction with an inflatable gasket which seals around the mouthpiece. Above the gasket there are channels which efficiently rinses the gasket and the filter with solvent. The user is aided by the station with functions such as dose actuation, solvent delivery, sample agitation and dose collector cleaning, after which the user collects the sample manually. The process is ended with an automated drying procedure. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Turbuhaler ® is one of the devices on this product category, see also Ellipta ® implementation, but can be made for essentially all devices upon customer request. See also attached flyer for in-depth information.
-


Mixing Inlet - Air-Flow Moderating Inlet (AMI)
The purpose of this product is inhaler testing aimed at achieving greater clinical relevance when testing dry-powder inhalers. Much like traditional mixing inlets, this product allows the user to maintain a constant flow rate into the cascade impactor with any chosen, time-varying flow rate through the DPI itself. We call it the Air-Flow Moderating Inlet (AMI)…a new friend of the inhaler testing community.
-

Dose Station Ellipta ®
This is our dedicated delivered dose uniformity test station for Ellipta ®, using the brilliant impinger filter dose collector. The latter is washable in-situ and can be ready for the next dose as part of the wet chemistry work-up procedure. The user works with the equipment in a fixed process which starts by the user placing the device in a nest which mates with the dose collector inlet. Thereafter aided by the station with functions such as dose actuation, solvent delivery, sample agitation and after which the user collects the sample manually. The process is ended with an automated drying procedure. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Ellipta ® is one of the devices on this product category, see also Turbuhaler ® implementation , but can be made for essentially all devices upon customer request. See also attached flyer for more information for in-depth information.
-
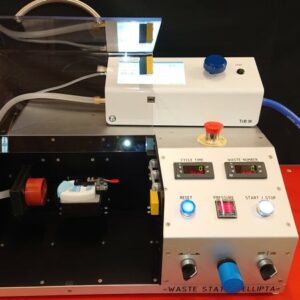
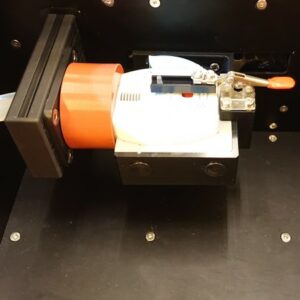
Waste Station Ellipta
This is our dedicated waste station for Ellipta. The user puts the Ellipta in a nest and closes a safety cover. The station then automatically mates the inhaler with the mouthpiece adapter, withdraws dose and then reverts. The process is repeated according to a user defined number, with selectable delay-time in between. The doses are collected on a high-capacity filter, with a holding capacity of about 10 g. The disposable filter is then safely and easily replaced. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Can be equipped with anti-static-device. See here for more information.
-


Induvidually Controlled Dissolution Baths
Dissolution bath with four separate vessels with stirrer motors. The stirrers are controlled individually, in terms of start (CW/CCW), stop and speed adjustment. Speed is adjustable between 20-300rpm and actual speed for each stirrer is presented digitally. The motors rest on hinges for better access when preparing the samples in the vessels. Stirrer shaft diameter is 10mm. Immersion heater is centrally positioned in the common water bath. The bath has ample lightning and it is possible to inspect the vessels underneath.
-


Nasal Spray Actuator
Take the worries out of Nasal Spray testing with FIA’s configurable NASAL SPRAY ACTUATOR. This actuation system enables the user to define multiple actuation methods. The nasal actuator measures the stroke travel distance and the force constantly, most notably end force at actuation. The velocity can be calculated from the distance and the time, recorded by the software.
The nasal actuator control box can be used to set number of actuations for dosing, rest time between each actuation, number of waste dose etc. The equipment can be easily adapted to most device geometries. The compact and open design makes it perfect to integrate with dose collection on filter, and with optical techniques for determination of, e.g., spray pattern, plume geometry (laser pulsed camera) and droplet size (laser diffraction). We can guide you to the best possible solution based on our historic implementations.
Main benefits:
- Control pivotal parameters affecting the dose and the spray
- Release technical staff from routine functions and potential ergonomic strain
- Elucidate and record actuations in documented fashion
The regulatory guidelines which are applicable:
- US (FDA) – Guidance for Industry: Bioavailability and Bioequivalence for Nasal Aerosols and Nasal Sprays for Local Action, Draft 2003.
- US (FDA) – Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products CMC Guidance, Draft 2002.
- EU (EMEA) – Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products, EMEA 2006.
-

Assuring Used Impactor Nozzle Quality
The ability to self-manage impactor nozzle quality is an important benefit for FIA customers who already own the FIA TrB III trigger box in combination with a speciality lid. FIA is now offering these measurements to customers who send their impactors -- either NGI or ACI -- to FIA for the same nozzle testing! FIA guarantees impactors sent to us for pressure drop measurement will be at FIA’s lab no more than five days! For the upper stages, we have rated pin gages by which we can measure if the stage nozzles are within specification. Pressure drop and dimensional checks will come with a certificate. In our machine shop we can also repair impactors and individual stages after agreement with the customer. The new FIA service, available to all impactor users, is MUCH FASTER and LESS expensive than optical inspection!! TRY IT NOW; you’ll be glad you did!!! For more technical understanding, press this link to the text that explains the link between dP, optical stage mensuration, and nozzle quality. If you are looking for the NGI service click here
-

Assuring Used Impactor Nozzle Quality
The ability to self-manage impactor nozzle quality is an important benefit for FIA customers who already own the FIA TrB III trigger box in combination with a speciality lid. FIA is now offering these measurements to customers who send their impactors -- either NGI or ACI -- to FIA for the same nozzle testing! FIA guarantees impactors sent to us for pressure drop measurement will be at FIA’s lab no more than five days! For the upper stages, we have rated pin gages by which we can measure if the stage nozzles are within specification. Pressure drop and dimensional checks will come with a certificate. In our machine shop we can also repair impactors and individual stages after agreement with the customer. The new FIA service, available to all impactor users, is MUCH FASTER and LESS expensive than optical inspection!! TRY IT NOW; you’ll be glad you did!!! For more technical understanding, press this link to the text that explains the link between dP, optical stage mensuration, and nozzle quality. If you are looking for the ACI service click here
-


Automated Salt Deposition Monitor (ASDM)
Insulator flashover happens when soluble and/or non-soluble contaminants cover the insulator surface which results in a reduction of the surface resistance [1]. FIA’s ASDM helps to predict the contamination level on the outdoor ceramic insulators surface. This can help as a mean to warn overhead lines operators about the advent of insulator flashover.
The ASDM consists of an equipment with a pilot insulator exposed to the same condition as the operational insulators in a, e.g., converter station. Many stations are situated in demanding areas, typically close to a shore line. By frequently automatically monitoring the Equivalent Salt Deposit Density (ESDD) it helps the operators to understand the build-up of contaminants on the insulator surface. The ASDM also measures wind and wind direction, enabling it to give a prognosis of potential flash over conditions.
The equipment is class IP64 and all outdoor parts are compatible with the demanding environment, such acid proof steel AISI 316, equivalent to corrosivity class C5-M.
The user interface for monitoring and control may be positioned remotely in an adjacent building.
The equipment is a modernized version of historical constructions installed at, e.g., nuclear power plants. FIA’s equipment has passed detailed technical reviews by demanding customers and our service team has passed the high security screens and made installations at restricted locations.
Request a quote or send a line to i@fia.se and we can tell more.
1. Abdelrahman K. Abouzeid, Ayman El-Hag & Khaled Assaleh (2018) Equivalent Salt Deposit Density Prediction of Silicone Rubber Insulators Under Simulated Pollution Conditions, Electric Power Components and Systems, 46:10, 1123-1133, DOI: 10.1080/15325008.2018.1488303
-

Andersen Blank Stage
To achieve an even distribution of a dose from an OINDP on a filter for dissolution testing [1], a blank stage is introduced next to the filter stage on the Andersen impactor. The blank stage (lower part in the picture) fits to the ordinary Andersen stage (upper part in the picture).
1. Sitaram P. Velagaa, Jelena Djuris, Sandra Cvijic, Stavroula Rozou, Paola Russo, Gaia Colombo Alessandra Rossi; European Journal of Pharmaceutical Sciences, ISSN: 0928-0987, Vol: 113, Page: 18-28
-


Automated Waste Station Turbuhaler/ICORes
can be customised according to your device, the example shown here is for Turbuhaler/ICORes. The station can be made to withdraw doses in different angles, i.e., different orientation of the device, to overcome “memory effects” of reservoir inhalers. Actuation can be by push, pull or as shown here, with a twist. The doses are collected on a high-capacity filter, with a holding capacity of about 10 g. The disposable filter is then safely and easily replaced. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Can be equipped with anti-static-device. See here for more information.
-

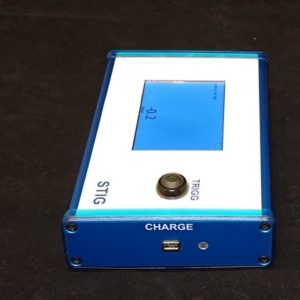
This FIA product addresses the dynamics of testing dry-powder inhalers (DPIs). Compendial methods of testing DPIs call for an abrupt start and abrupt end to the air flow. An attribute aimed at representing the way a patient uses a DPI. The starting and stopping of the air flow introduces time-variant conditions in the test equipment (cascade impactor) and in the device itself. Experimental and computational studies of these conditions are continuing to elucidate the important characteristics of the device and test system that should be known and controlled to establish a sound quality control test for products that are or are about to be registered. FIA's latest instrument known as Stig puts in users hands the most important measure of the time-dependent air flow start-up the rise-time. It is important that the rise-time is known and under control when testing inhalable devices. Russell-Graham and colleagues1showed that the fine particle dose increases as the rise time decreases. Previous and subsequent theoretical analyses point out reasons for this effect.2, 3 For established products quality control (QC) testing requires knowing that the rise-time remains in a specified range...now possible with Stig. For GMP QC Stig can be locked to acquire and present the rise-time according to a defined and validated method. Since patients do generate different inhalation air-flow profiles it is also important during the development of a new drug product to adjust the prototype devices to exhibit a sensible rise-time close to what will take place in patient use. This mindset is equally important for DPIs and for breath-actuated MDI devices. Stig also makes possible the recording of air-flow profiles so that they can be reproduced on a breathing simulator (such as F-SIG 6300) for studying nebulizers. Versatile and user-friendly. Best-in-class - that is the new Stig from FIA.
Key features of Stig Restricted Version:
- Rise-time measurement 0.1-1 s using a thermal flow meter
- Average rise-time from a series of measurements
- Touch-screen which displays a graph of flow vs. time, rise-time and the final flow
- Battery powered
- Optional IQ/OQ and quality certificate for the regulated industry
Download the pdf Rise-time Measurement Instrument Stig
- Russell-Graham, D., A. Cooper, B. Stobbs, E. McAulay, H. Bogard, V. Heith, E. Monsallier, "Further Evaluation of the Fast-Screening Impactor for Determining Fine-Particle Fraction of Dry Powder Inhalers, "Drug Delivery to the Lung, December 8 to 10, 2010, Edinburgh, Scotland.
- Roberts, D. L., M. Chiruta, "Transient Impactor Behavior during the Testing of Dry-Powder Inhalers via Compendial Methods, "Drug Delivery to the Lung 18, The Aerosol Society, Edinburgh, Scotland, December 13-14, 2007.
- Versteeg, H., P. Zhao, C. Blatchford, M. Copley, D. L. Roberts, J. P. Mitchell, "A Computational Fluid Dynamics (CFD) Model of the Start-Up Kinetics of the Andersen Cascade Impactor (ACI), "Drug Delivery to the Lung, Aerosol Society, Edinburgh, Scotland, December 9-11 2015; pages 18-21.