Description
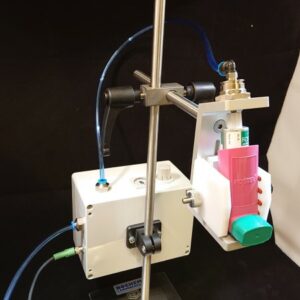
Nasal Spray Actuator
Take the worries out of Nasal Spray testing with
FIA’s configurable NASAL SPRAY ACTUATOR. This
actuation system enables the user to define multiple
actuation methods. The nasal actuator measures the
stroke travel distance and the force constantly, most
notably end force at actuation. The velocity can be
calculated from the distance and the time, recorded
by the software.
The nasal actuator control box can be used to set number
of actuations for dosing, rest time between each actuation,
number of waste dose etc. The equipment can be easily
adapted to most device geometries. The compact and open
design makes it perfect to integrate with dose collection on
filter, and with optical techniques for determination of, e.g.,
spray pattern, plume geometry (laser pulsed camera) and
droplet size (laser diffraction). We can guide you to the best
possible solution based on our historic implementations.
Main benefits:
- Control pivotal parameters affecting the dose
and the spray - Release technical staff from routine functions
and potential ergonomic strain - Elucidate and record actuations in documented
fashion
The regulatory guidelines which
are applicable:
- US (FDA) – Guidance for Industry: Bioavailability and
Bioequivalence for Nasal Aerosols and Nasal Sprays
for Local Action, Draft 2003. - US (FDA) – Nasal Spray and Inhalation Solution,
Suspension, and Spray Drug Products CMC Guidance,
Draft 2002. - EU (EMEA) – Guideline on the Pharmaceutical Quality
of Inhalation and Nasal Products, EMEA 2006.