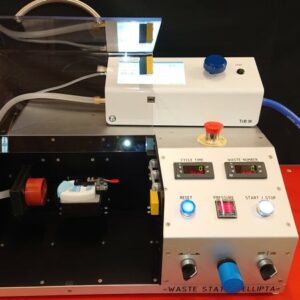
TrB III Trigger Box Delta – All-in-One Flow Controller
The TrB III Trigger Box ensures compliance with standard pharmacopeial methods, both recording and storing key system parameters, including the actual flow rate and run duration. Many inhaler test methods rely on critical flow conditions across the flow control valve, aiming to ensure the same flow rate on each test. But the TrB III does more – actually measures the flow of each test – so, there are no assumptions.
A calibrated laminar flow element (LFE) internal to each TrB III enables the user to set the flow rate at the beginning of a test sequence; with this LFE, the TrB III then records the flow rate of each test, ensuring against drift, leaks, and other non-ideal behavior that may introduce variability in test results. The TrB III also records the other more traditional run-time parameters, such as the test duration, the pressure drop across the inhaler device (P1), and the flow control pressure ratio (P3/P2, critical flow if ≤ 0.5). The Delta version can measure pressure drop over, e.g., individual impactor stages to detect blockage, using additional internal sensors.1
Additional user-friendly functions are leak checking and synchronized device actuation by using the integrated output port. Device actuation enables the flow to start simultaneously with dose actuation of a metered-dose inhaler, allowing a user- defined, fixed flow volume for MDI total dose testing.
Download AB Fia folder Trigger Box TrB III.
Key features are:
- Flow actuation: 0-60 min, 0.1s resolution
- Actuation counter: Resettable 0-999
- Foot switch: Yes
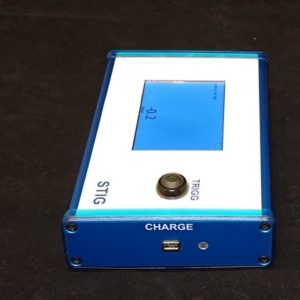
- Display: 7” touch
- P1 measurement: Yes, 0-16 kPa
- P3/P2 measurement: Yes
- Flow measurement: Calibrated 0 – 120 l/min (operating range possibly higher)
- dP (e.g. stage dP): Yes, high precision 0-6 kPa
- Automatic leak test: Yes
- Printable data: Prints new actuations continuously or print all actuations from reset.
- Date/time of first dose
- Instrument ID
- Instrument ver
- Flow ”on” time
- External relay timing
- Atmospheric pressure
- Dose number
- P1 and flow
- P3/P2 (if < 0.5)
- dP (stage pressure drop)
- Relay output for actuation of external equipment: Yes, configurable timing of output relative vacuum opening.
- Displayed history of recent actuation data: All actuations from reset.
- Interfaces:
- Relay output for actuation of external equipment
- Foot switch actuator
- USB for CSV export
- Dimensions (cm): 34x13x13
Download the pdf TrB III – All in one Flow Controller