Female adapter to the 22 mm (male) side of a ISO 22 standard filter. Comes with a barbed fitting for vacuum hose. The adapter will also fit a, e.g., TSI 22 mm flow meter.
-


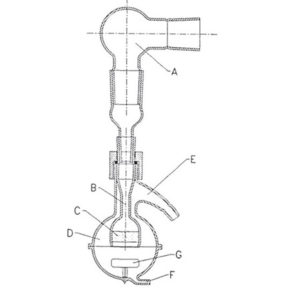
A convenient way to introduce a P1 tap to measure the pressure drop over a device according to pharmacopoeia. See example when applied to a waste station.
-

Andersen Blank Stage
To achieve an even distribution of a dose from an OINDP on a filter for dissolution testing [1], a blank stage is introduced next to the filter stage on the Andersen impactor. The blank stage (lower part in the picture) fits to the ordinary Andersen stage (upper part in the picture).
1. Sitaram P. Velagaa, Jelena Djuris, Sandra Cvijic, Stavroula Rozou, Paola Russo, Gaia Colombo Alessandra Rossi; European Journal of Pharmaceutical Sciences, ISSN: 0928-0987, Vol: 113, Page: 18-28
-


Automated Waste Station Turbuhaler/ICORes
can be customised according to your device, the example shown here is for Turbuhaler/ICORes. The station can be made to withdraw doses in different angles, i.e., different orientation of the device, to overcome “memory effects” of reservoir inhalers. Actuation can be by push, pull or as shown here, with a twist. The doses are collected on a high-capacity filter, with a holding capacity of about 10 g. The disposable filter is then safely and easily replaced. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Can be equipped with anti-static-device. See here for more information.
-

Patient Realistic Inhaler Testing
The F-SIG 6300 by AB FIA is a breathing profile generator that is capable of replaying complex breathing patterns. Thanks to a novel multi-cylinder design F-SIG 6300 enables generation of a number of clinical relevant patterns. F-SIG 6300 is not intended for clinical use on humans. The areas of operation are for instance: Testing of pharmaceutical inhalers and nebulizers with human breathing patterns. Characterization of cigarettes (smokers profiles). Rodent profiles and general respiratory research that includes very controlled breathing patterns.
User Friendly and Versatile
The F-SIG 6300 is operator-friendly and comes with a touch panel. Profiles, possibly recorded by FIA's Metivent software, are handled and modified in a spreadsheet (e.g. Excel) and the profile library is downloaded to the F-SIG 6300. The operator can thereafter, from the library of profiles, select desired profile on the panel and start the replay of the profile.
The Equipment:
F-SIG 6300 consists of a breathing profile generator built into a box with transparent plastic covers and placed on wheels. The control system of the multi-cylinder package is configured and operated by the user through the touch panel.
Capacity large volume: 0 - 6.3 liters
Capacity small volume: 0 - 0.1 liters
Voltage: 95 - 255VAC, 50 - 60Hz, 6.5A
Dimensions HxWxD: 380x1036x480 mm, Touch Panel: 80x280x170 mmDownload the pdf Patient Realistic Inhaler Testing
-

Dose Station Ellipta ®
This is our dedicated delivered dose uniformity test station for Ellipta ®, using the brilliant impinger filter dose collector. The latter is washable in-situ and can be ready for the next dose as part of the wet chemistry work-up procedure. The user works with the equipment in a fixed process which starts by the user placing the device in a nest which mates with the dose collector inlet. Thereafter aided by the station with functions such as dose actuation, solvent delivery, sample agitation and after which the user collects the sample manually. The process is ended with an automated drying procedure. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Ellipta ® is one of the devices on this product category, see also Turbuhaler ® implementation , but can be made for essentially all devices upon customer request. See also attached flyer for more information for in-depth information.
-

Dose Station Turbuhaler ®
This is our dedicated delivered dose uniformity test station for Turbuhaler ®, using the brilliant impinger filter dose collector. The latter is washable in-situ and can be ready for the next dose as part of the wet chemistry work-up procedure. The user works with the equipment in a fixed process which starts by the user placing the device in an inlet which mates with the dose collector. The inlet is a highly sophisticated construction with an inflatable gasket which seals around the mouthpiece. Above the gasket there are channels which efficiently rinses the gasket and the filter with solvent. The user is aided by the station with functions such as dose actuation, solvent delivery, sample agitation and dose collector cleaning, after which the user collects the sample manually. The process is ended with an automated drying procedure. The integration with FIA’s TriggerBox III gives the user full control of the flow process and relevant data recorded on file or printer. Turbuhaler ® is one of the devices on this product category, see also Ellipta ® implementation, but can be made for essentially all devices upon customer request. See also attached flyer for in-depth information.
-
 With this manifold you can regulate the flow of both vacuum (negative pressure) and pressurised flow while keeping the lab bench tidy. Typically used in combination with mixing inlet (Mixing Inlet - Air-Flow Moderating Inlet (AMI) - AB FIA Online Store) where a precise flow duration might not be needed. For the latter please see our line of critical flow controllers: Flow and Actuation Control - AB FIA Online Store It can come assembled with fittings and tubing and shut-off valves for vacuum and pressurised air. Describe your needs and we will come back with an offer.
With this manifold you can regulate the flow of both vacuum (negative pressure) and pressurised flow while keeping the lab bench tidy. Typically used in combination with mixing inlet (Mixing Inlet - Air-Flow Moderating Inlet (AMI) - AB FIA Online Store) where a precise flow duration might not be needed. For the latter please see our line of critical flow controllers: Flow and Actuation Control - AB FIA Online Store It can come assembled with fittings and tubing and shut-off valves for vacuum and pressurised air. Describe your needs and we will come back with an offer. -


Automated Delivered Dose uniformity (DDU) and Waste station. A large number of inhalers is loaded into a magazine and the controller works down a list of operations such as delivered dose testing and waste dosing (between periods of the dosing regime). The technology builds on well-established process control equipment from, e.g., Siemens and Festo, why it sits well withing the customers existing support and maintenance organisation. Can be customized to your device. Read more here.
-


Glass Frit Impinger DUSA
Used as an alternative to the DUSA after proper equivalence testing (ask us how!). Used by many of the leading companies within OINDP testing. This filter comes in various forms but adheres to the principles which was published in a USP stimuli article 1993. 1 It lends itself to high-capacity testing and automation since it is washable with good recovery and minor carry-over.
- 1. Hugosson, S., J. Lindberg, T. Lööf, B. Olsson, ”Proposals for Standardized Testing of Powder Preparations for Inhalation”, Pharm Forum, Vol 19-3, 5458-66, 1993
-

Assuring Used Impactor Nozzle Quality
The ability to self-manage impactor nozzle quality is an important benefit for FIA customers who already own the FIA TrB III trigger box in combination with a speciality lid. FIA is now offering these measurements to customers who send their impactors -- either NGI or ACI -- to FIA for the same nozzle testing! FIA guarantees impactors sent to us for pressure drop measurement will be at FIA’s lab no more than five days! For the upper stages, we have rated pin gages by which we can measure if the stage nozzles are within specification. Pressure drop and dimensional checks will come with a certificate. In our machine shop we can also repair impactors and individual stages after agreement with the customer. The new FIA service, available to all impactor users, is MUCH FASTER and LESS expensive than optical inspection!! TRY IT NOW; you’ll be glad you did!!! For more technical understanding, press this link to the text that explains the link between dP, optical stage mensuration, and nozzle quality. If you are looking for the NGI service click here
-

Assuring Used Impactor Nozzle Quality
The ability to self-manage impactor nozzle quality is an important benefit for FIA customers who already own the FIA TrB III trigger box in combination with a speciality lid. FIA is now offering these measurements to customers who send their impactors -- either NGI or ACI -- to FIA for the same nozzle testing! FIA guarantees impactors sent to us for pressure drop measurement will be at FIA’s lab no more than five days! For the upper stages, we have rated pin gages by which we can measure if the stage nozzles are within specification. Pressure drop and dimensional checks will come with a certificate. In our machine shop we can also repair impactors and individual stages after agreement with the customer. The new FIA service, available to all impactor users, is MUCH FASTER and LESS expensive than optical inspection!! TRY IT NOW; you’ll be glad you did!!! For more technical understanding, press this link to the text that explains the link between dP, optical stage mensuration, and nozzle quality. If you are looking for the ACI service click here
-
 Inhaler mouthpiece adapter IP blue to impactor induction port.
Inhaler mouthpiece adapter IP blue to impactor induction port.
Material: Blue silicone (harder and more durable). FIA also sells impactors and induction ports. -
 Inhaler mouthpiece adapter IP red to impactor induction port.
Inhaler mouthpiece adapter IP red to impactor induction port.
Material: Red silicone (softer and more "forgiving"). FIA also sells impactors and induction ports. -
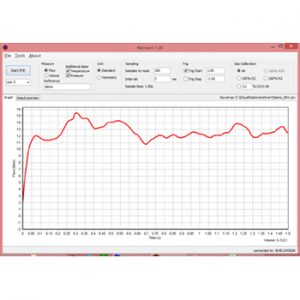

Metivent Software - A Companion to TSI Flowmeters
Metivent is a Windows application for recording air flow measurements from TSI flowmeters (e.g. TSI4040). A standard Windows computer with the installed Metivent software connects to the flowmeter via a USB-serial converter. The acquisition of data from the flowmeter is then controlled by the software. Once started the graph is updated continuously during measurement.
- The software can be used in several modes and configurations.
- Start trig (wait to start until the flow has reached the start trigger level)
- End trig (sample data until stop trigger condition)
- Gas calibration
- Number of samples to collect if not using trig
- Standard or volumetric flow
- Sampling rate 1-1000/s
Measurements can be conveniently saved to CSV files and opened in Excel for further data analyses and presentations. The instrument is pivotal when recording inhalations profiles for lung simulation studies (such as F-SIG 6300). The file presents flow and pressure and temperature along with instrument data such as serial number and calibration date and currently used settings.
Download the pdf ABFIA Metivent Software
-


Mixing Inlet - Air-Flow Moderating Inlet (AMI)
The purpose of this product is inhaler testing aimed at achieving greater clinical relevance when testing dry-powder inhalers. Much like traditional mixing inlets, this product allows the user to maintain a constant flow rate into the cascade impactor with any chosen, time-varying flow rate through the DPI itself. We call it the Air-Flow Moderating Inlet (AMI)…a new friend of the inhaler testing community.