-

Trigger Box Model I – Basic
This model gives the analyst the basic functionality in a very compact and easily managed format.
Key features are:
- Flow actuation: 0-99 min, 0.1s resolution
- Actuation counter: Resettable, 8 digits
- Foot switch: Optional
- Display: 4 and 8 digits
- P1 Measurement: No
- P3/P2 Measurement: No
- Flow Measurement: No
- dP (e.g. stage dP): No
- Printable data: No
- Relay output for actuation of external equipment: No
- Displayed history of recent actuation data: No
- USB memory stick export: No
- Automatic leak test: No
- Dimensions (cm): 23x13x13
-

Trigger Box Model II – Critical Flow Controller
This model builds on the functions of Model I with the addition of differential pressure transducers for measuring and displaying pressure at P1, P2, and P3.
Key features are:
- Flow actuation: 0-60 min, 0.1s resolution
- Actuation counter: Resettable 0-999
- Foot switch: Yes
- Display: 4.3” touch
- P1 Measurement: Yes, 0-16 kPa
- P3/P2 Measurement: Yes
- Flow Measurement: No
- dP (e.g. stage dP): No
- Printable data: Prints new actuations continuously
- Flow ”on” time
- Dose number
- P1
- P3/P2 (if < 0.5)
- Relay output for actuation of external equipment: No
- Displayed history of recent actuation data: Last 3 actuations
- USB memory stick export: No
- Automatic leak test: No
- Dimensions (cm): 23x13x13
-


TrB III Trigger Box Delta – All-in-One Flow Controller
The TrB III Trigger Box ensures compliance with standard pharmacopeial methods, both recording and storing key system parameters, including the actual flow rate and run duration. Many inhaler test methods rely on critical flow conditions across the flow control valve, aiming to ensure the same flow rate on each test. But the TrB III does more – actually measures the flow of each test – so, there are no assumptions.
A calibrated laminar flow element (LFE) internal to each TrB III enables the user to set the flow rate at the beginning of a test sequence; with this LFE, the TrB III then records the flow rate of each test, ensuring against drift, leaks, and other non-ideal behavior that may introduce variability in test results. The TrB III also records the other more traditional run-time parameters, such as the test duration, the pressure drop across the inhaler device (P1), and the flow control pressure ratio (P3/P2, critical flow if ≤ 0.5). The Delta version can measure pressure drop over, e.g., individual impactor stages to detect blockage, using additional internal sensors.1
Additional user-friendly functions are leak checking and synchronized device actuation by using the integrated output port. Device actuation enables the flow to start simultaneously with dose actuation of a metered-dose inhaler, allowing a user- defined, fixed flow volume for MDI total dose testing.
Download AB Fia folder Trigger Box TrB III.
Key features are:
- Flow actuation: 0-60 min, 0.1s resolution
- Actuation counter: Resettable 0-999
- Foot switch: Yes
- Display: 7” touch
- P1 measurement: Yes, 0-16 kPa
- P3/P2 measurement: Yes
- Flow measurement: Calibrated 0 – 120 l/min (operating range possibly higher)
- dP (e.g. stage dP): Yes, high precision 0-6 kPa
- Automatic leak test: Yes
- Printable data: Prints new actuations continuously or print all actuations from reset.
- Date/time of first dose
- Instrument ID
- Instrument ver
- Flow ”on” time
- External relay timing
- Atmospheric pressure
- Dose number
- P1 and flow
- P3/P2 (if < 0.5)
- dP (stage pressure drop)
- Relay output for actuation of external equipment: Yes, configurable timing of output relative vacuum opening.
- Displayed history of recent actuation data: All actuations from reset.
- Interfaces:
- Relay output for actuation of external equipment
- Foot switch actuator
- USB for CSV export
- Dimensions (cm): 34x13x13
Download the pdf TrB III – All in one Flow Controller
-


Volumetric dispenser with a programmable volume in the interval of 10 to 1100 ml. The volume may be repeated to pump several liters in portions. This function may be used for instance to repeatedly fill vessels.
On the picture is shown a product version with a stand. It may also be mounted on a stainless lab trolley or with the lower part under a table.
All parts in liquid contact are corrosion resistant, allowing handling of aggressive liquids and solvents. It is also available for dilution purposes with extra valves.
Easy to operate via touch panel. No PC is needed. Just type in the desired volume in ml and the dispenser will withdraw the volume from the container and dispense it from the outlet.
Different liquids will need different withdrawal and infusion (dispensing) speeds which is easily changed through the panel.
Exact dosage of liquid volumes is in the range of < 10 – 1100 ml. The set volume can be repeated, thus it is also possible to dispense for instance 999 x 1100 ml.
Standard deviation less than 0.02.
Volume accuracy and precision: fault < 0.5 ml in the interval 30 – 300 ml and in the interval 300 – 1100 ml < 0.4%.
The concept can be made in other configurations, such as compact models with shorter but broader cylinders, when a slighty less accurate delivery is acceptable. Lower volume ranges, e.g., 500 ml are also available.
-


The Controlled Pump is designed to run 32 different pump programs that are easy selectable by two thumb wheels on the front panel. A program consists of one infusion motion and one withdrawal motion which are both configurable with stroke length and speed.
Start a selected pump program by press of the green “START/STOP” push button at the front panel. The pump will start infuse with parameters from the selected pump program. When the pump reach the programmed end position, it will immediately withdraw and then infuse and so on, as long as the latched push button is in its inner position.
Specifications
Dimensions: 122 x 140 x 350 mm. (WxDxH)
Weight: 4,4 kg.
Power supply: External power supply, 24 VDC 2,5 A
Glass syringe connection for external equipment: Luer Lock
Maximum infusion/withdrawal volume: 20 ml.
Maximum infusion/withdrawal rate (measured directly against atmosphere) 50 ml/s.
Lowest infusion/withdrawal rate (measured directly against atmosphere) 6 ml/s. -

Key features:
- Can be operated in a series
- Very accurate with a repeatability of 0.04% RSD
- Simplicity in design
- Long life time of dispensing equipment
- Constructed of glass and teflon
- Suitable for integration in robotic systems
- Can be ordered on a mobile platform for best working environment
- Full volume each stroke
- Impact time 10-20 sec
- No PLC nor computer dependent, but can be easily connected
All dispensers require compressed air of 6-8 bar.
-

Key features:
- Can be operated in a series
- Very accurate with a repeatability of 0.04% RSD
- Simplicity in design
- Long life time of dispensing equipment
- Constructed of glass and teflon
- Suitable for integration in robotic systems
- Can be ordered on a mobile platform for best working environment
- Full volume each stroke
- Impact time 10-20 sec
- No PLC nor computer dependent, but can be easily connected
All dispensers require compressed air of 6-8 bar.
-


This FIA product addresses the dynamics of testing dry-powder inhalers (DPIs). Compendial methods of testing DPIs call for an abrupt start and abrupt end to the air flow. An attribute aimed at representing the way a patient uses a DPI. The starting and stopping of the air flow introduces time-variant conditions in the test equipment (cascade impactor) and in the device itself. Experimental and computational studies of these conditions are continuing to elucidate the important characteristics of the device and test system that should be known and controlled to establish a sound quality control test for products that are or are about to be registered. FIA's latest instrument known as Stig puts in users hands the most important measure of the time-dependent air flow start-up the rise-time. It is important that the rise-time is known and under control when testing inhalable devices. Russell-Graham and colleagues1showed that the fine particle dose increases as the rise time decreases. Previous and subsequent theoretical analyses point out reasons for this effect.2, 3 For established products quality control (QC) testing requires knowing that the rise-time remains in a specified range...now possible with Stig. For GMP QC Stig can be locked to acquire and present the rise-time according to a defined and validated method. Since patients do generate different inhalation air-flow profiles it is also important during the development of a new drug product to adjust the prototype devices to exhibit a sensible rise-time close to what will take place in patient use. This mindset is equally important for DPIs and for breath-actuated MDI devices. Stig also makes possible the recording of air-flow profiles so that they can be reproduced on a breathing simulator (such as F-SIG 6300) for studying nebulizers. Versatile and user-friendly. Best-in-class - that is the new Stig from FIA.
Key features of Stig:
- Rise-time measurement 0.1-1 s using a thermal flow meter
- Average rise-time from a series of measurements
- Touch-screen which displays a graph of flow vs. time, rise-time and the final flow
- Printed records of the measurement with optional printer. Has been validated together with Mettler-Toledo printers, customer can use own printers (e.g. from lab balances)
- Rise-time profile saved to USB-memory
- Date and time
- Battery powered
- Optional IQ/OQ and quality certificate for the regulated industry
Download the pdf Rise-time Measurement Instrument Stig
- Russell-Graham, D., A. Cooper, B. Stobbs, E. McAulay, H. Bogard, V. Heith, E. Monsallier, "Further Evaluation of the Fast-Screening Impactor for Determining Fine-Particle Fraction of Dry Powder Inhalers, "Drug Delivery to the Lung, December 8 to 10, 2010, Edinburgh, Scotland.
- Roberts, D. L., M. Chiruta, "Transient Impactor Behavior during the Testing of Dry-Powder Inhalers via Compendial Methods, "Drug Delivery to the Lung 18, The Aerosol Society, Edinburgh, Scotland, December 13-14, 2007.
- Versteeg, H., P. Zhao, C. Blatchford, M. Copley, D. L. Roberts, J. P. Mitchell, "A Computational Fluid Dynamics (CFD) Model of the Start-Up Kinetics of the Andersen Cascade Impactor (ACI), "Drug Delivery to the Lung, Aerosol Society, Edinburgh, Scotland, December 9-11 2015; pages 18-21.
-
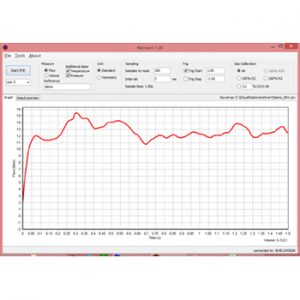

Metivent Software - A Companion to TSI Flowmeters
Metivent is a Windows application for recording air flow measurements from TSI flowmeters (e.g. TSI4040). A standard Windows computer with the installed Metivent software connects to the flowmeter via a USB-serial converter. The acquisition of data from the flowmeter is then controlled by the software. Once started the graph is updated continuously during measurement.
- The software can be used in several modes and configurations.
- Start trig (wait to start until the flow has reached the start trigger level)
- End trig (sample data until stop trigger condition)
- Gas calibration
- Number of samples to collect if not using trig
- Standard or volumetric flow
- Sampling rate 1-1000/s
Measurements can be conveniently saved to CSV files and opened in Excel for further data analyses and presentations. The instrument is pivotal when recording inhalations profiles for lung simulation studies (such as F-SIG 6300). The file presents flow and pressure and temperature along with instrument data such as serial number and calibration date and currently used settings.
Download the pdf ABFIA Metivent Software
-

Training and IQ Metivent Software. Can be done remote via Skype or on-site. Request preference. The IQ results in a wet-ink protocol. The user gets a license to copyrighted text for e.g. internal maintenance work. Configurable for inhaler actuation. Configurable for camera sync. Configurable for load cell.
-

Patient Realistic Inhaler Testing
The F-SIG 6300 by AB FIA is a breathing profile generator that is capable of replaying complex breathing patterns. Thanks to a novel multi-cylinder design F-SIG 6300 enables generation of a number of clinical relevant patterns. F-SIG 6300 is not intended for clinical use on humans. The areas of operation are for instance: Testing of pharmaceutical inhalers and nebulizers with human breathing patterns. Characterization of cigarettes (smokers profiles). Rodent profiles and general respiratory research that includes very controlled breathing patterns.
User Friendly and Versatile
The F-SIG 6300 is operator-friendly and comes with a touch panel. Profiles, possibly recorded by FIA's Metivent software, are handled and modified in a spreadsheet (e.g. Excel) and the profile library is downloaded to the F-SIG 6300. The operator can thereafter, from the library of profiles, select desired profile on the panel and start the replay of the profile.
The Equipment:
F-SIG 6300 consists of a breathing profile generator built into a box with transparent plastic covers and placed on wheels. The control system of the multi-cylinder package is configured and operated by the user through the touch panel.
Capacity large volume: 0 - 6.3 liters
Capacity small volume: 0 - 0.1 liters
Voltage: 95 - 255VAC, 50 - 60Hz, 6.5A
Dimensions HxWxD: 380x1036x480 mm, Touch Panel: 80x280x170 mmDownload the pdf Patient Realistic Inhaler Testing



















