Set screw with PTFE bushing for Axis < July-2010, 5pc.
-
 Used for creating a continuous infusion system, dual infusion system, or one of the other 2 pump automation modes.
Used for creating a continuous infusion system, dual infusion system, or one of the other 2 pump automation modes.
Establishes a communications link between two pumps using the RS-232 serial ports on the pumps.
Replaces cable CBL-TTL-1, unless the use of the TTL ports for synchronization is preferred
Download User Manual -
 Allows full control of pump or other device from a computer
Allows full control of pump or other device from a computer
DB-9 adapter connects to 9-pin serial port (25-pin adapters available upon request)
Utilizes the RS-232 communications port of device
Quick and easy setup
Add to basket Details Allows full control of pump or other device from a computer
Allows full control of pump or other device from a computer
DB-9 adapter connects to 9-pin serial port (25-pin adapters available upon request)
Utilizes the RS-232 communications port of device
Quick and easy setup
Add to basket Details
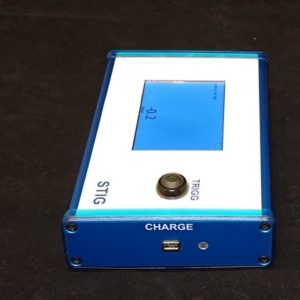
This FIA product addresses the dynamics of testing dry-powder inhalers (DPIs). Compendial methods of testing DPIs call for an abrupt start and abrupt end to the air flow. An attribute aimed at representing the way a patient uses a DPI. The starting and stopping of the air flow introduces time-variant conditions in the test equipment (cascade impactor) and in the device itself. Experimental and computational studies of these conditions are continuing to elucidate the important characteristics of the device and test system that should be known and controlled to establish a sound quality control test for products that are or are about to be registered. FIA's latest instrument known as Stig puts in users hands the most important measure of the time-dependent air flow start-up the rise-time. It is important that the rise-time is known and under control when testing inhalable devices. Russell-Graham and colleagues1showed that the fine particle dose increases as the rise time decreases. Previous and subsequent theoretical analyses point out reasons for this effect.2, 3 For established products quality control (QC) testing requires knowing that the rise-time remains in a specified range...now possible with Stig. For GMP QC Stig can be locked to acquire and present the rise-time according to a defined and validated method. Since patients do generate different inhalation air-flow profiles it is also important during the development of a new drug product to adjust the prototype devices to exhibit a sensible rise-time close to what will take place in patient use. This mindset is equally important for DPIs and for breath-actuated MDI devices. Stig also makes possible the recording of air-flow profiles so that they can be reproduced on a breathing simulator (such as F-SIG 6300) for studying nebulizers. Versatile and user-friendly. Best-in-class - that is the new Stig from FIA.
Key features of Stig Restricted Version:
- Rise-time measurement 0.1-1 s using a thermal flow meter
- Average rise-time from a series of measurements
- Touch-screen which displays a graph of flow vs. time, rise-time and the final flow
- Battery powered
- Optional IQ/OQ and quality certificate for the regulated industry
Download the pdf Rise-time Measurement Instrument Stig
- Russell-Graham, D., A. Cooper, B. Stobbs, E. McAulay, H. Bogard, V. Heith, E. Monsallier, "Further Evaluation of the Fast-Screening Impactor for Determining Fine-Particle Fraction of Dry Powder Inhalers, "Drug Delivery to the Lung, December 8 to 10, 2010, Edinburgh, Scotland.
- Roberts, D. L., M. Chiruta, "Transient Impactor Behavior during the Testing of Dry-Powder Inhalers via Compendial Methods, "Drug Delivery to the Lung 18, The Aerosol Society, Edinburgh, Scotland, December 13-14, 2007.
- Versteeg, H., P. Zhao, C. Blatchford, M. Copley, D. L. Roberts, J. P. Mitchell, "A Computational Fluid Dynamics (CFD) Model of the Start-Up Kinetics of the Andersen Cascade Impactor (ACI), "Drug Delivery to the Lung, Aerosol Society, Edinburgh, Scotland, December 9-11 2015; pages 18-21.


This FIA product addresses the dynamics of testing dry-powder inhalers (DPIs). Compendial methods of testing DPIs call for an abrupt start and abrupt end to the air flow. An attribute aimed at representing the way a patient uses a DPI. The starting and stopping of the air flow introduces time-variant conditions in the test equipment (cascade impactor) and in the device itself. Experimental and computational studies of these conditions are continuing to elucidate the important characteristics of the device and test system that should be known and controlled to establish a sound quality control test for products that are or are about to be registered. FIA's latest instrument known as Stig puts in users hands the most important measure of the time-dependent air flow start-up the rise-time. It is important that the rise-time is known and under control when testing inhalable devices. Russell-Graham and colleagues1showed that the fine particle dose increases as the rise time decreases. Previous and subsequent theoretical analyses point out reasons for this effect.2, 3 For established products quality control (QC) testing requires knowing that the rise-time remains in a specified range...now possible with Stig. For GMP QC Stig can be locked to acquire and present the rise-time according to a defined and validated method. Since patients do generate different inhalation air-flow profiles it is also important during the development of a new drug product to adjust the prototype devices to exhibit a sensible rise-time close to what will take place in patient use. This mindset is equally important for DPIs and for breath-actuated MDI devices. Stig also makes possible the recording of air-flow profiles so that they can be reproduced on a breathing simulator (such as F-SIG 6300) for studying nebulizers. Versatile and user-friendly. Best-in-class - that is the new Stig from FIA.
Key features of Stig:
- Rise-time measurement 0.1-1 s using a thermal flow meter
- Average rise-time from a series of measurements
- Touch-screen which displays a graph of flow vs. time, rise-time and the final flow
- Printed records of the measurement with optional printer. Has been validated together with Mettler-Toledo printers, customer can use own printers (e.g. from lab balances)
- Rise-time profile saved to USB-memory
- Date and time
- Battery powered
- Optional IQ/OQ and quality certificate for the regulated industry
Download the pdf Rise-time Measurement Instrument Stig
- Russell-Graham, D., A. Cooper, B. Stobbs, E. McAulay, H. Bogard, V. Heith, E. Monsallier, "Further Evaluation of the Fast-Screening Impactor for Determining Fine-Particle Fraction of Dry Powder Inhalers, "Drug Delivery to the Lung, December 8 to 10, 2010, Edinburgh, Scotland.
- Roberts, D. L., M. Chiruta, "Transient Impactor Behavior during the Testing of Dry-Powder Inhalers via Compendial Methods, "Drug Delivery to the Lung 18, The Aerosol Society, Edinburgh, Scotland, December 13-14, 2007.
- Versteeg, H., P. Zhao, C. Blatchford, M. Copley, D. L. Roberts, J. P. Mitchell, "A Computational Fluid Dynamics (CFD) Model of the Start-Up Kinetics of the Andersen Cascade Impactor (ACI), "Drug Delivery to the Lung, Aerosol Society, Edinburgh, Scotland, December 9-11 2015; pages 18-21.

Key features:
- Can be operated in a series
- Very accurate with a repeatability of 0.04% RSD
- Simplicity in design
- Long life time of dispensing equipment
- Constructed of glass and teflon
- Suitable for integration in robotic systems
- Can be ordered on a mobile platform for best working environment
- Full volume each stroke
- Impact time 10-20 sec
- No PLC nor computer dependent, but can be easily connected
All dispensers require compressed air of 6-8 bar.

Key features:
- Can be operated in a series
- Very accurate with a repeatability of 0.04% RSD
- Simplicity in design
- Long life time of dispensing equipment
- Constructed of glass and teflon
- Suitable for integration in robotic systems
- Can be ordered on a mobile platform for best working environment
- Full volume each stroke
- Impact time 10-20 sec
- No PLC nor computer dependent, but can be easily connected
All dispensers require compressed air of 6-8 bar.

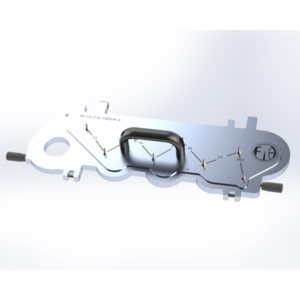
This precisely machined lid temporarily replaces that on the NGI and is used together with the highly versatile flow controller TrBIII Delta


Nasal Spray Actuator
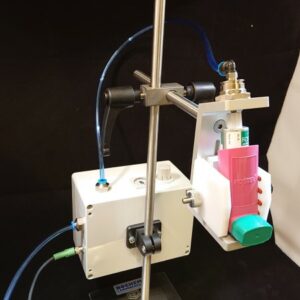
Take the worries out of Nasal Spray testing with FIA’s configurable NASAL SPRAY ACTUATOR. This actuation system enables the user to define multiple actuation methods. The nasal actuator measures the stroke travel distance and the force constantly, most notably end force at actuation. The velocity can be calculated from the distance and the time, recorded by the software.
The nasal actuator control box can be used to set number of actuations for dosing, rest time between each actuation, number of waste dose etc. The equipment can be easily adapted to most device geometries. The compact and open design makes it perfect to integrate with dose collection on filter, and with optical techniques for determination of, e.g., spray pattern, plume geometry (laser pulsed camera) and droplet size (laser diffraction). We can guide you to the best possible solution based on our historic implementations.
Main benefits:
- Control pivotal parameters affecting the dose and the spray
- Release technical staff from routine functions and potential ergonomic strain
- Elucidate and record actuations in documented fashion
The regulatory guidelines which are applicable:
- US (FDA) – Guidance for Industry: Bioavailability and Bioequivalence for Nasal Aerosols and Nasal Sprays for Local Action, Draft 2003.
- US (FDA) – Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products CMC Guidance, Draft 2002.
- EU (EMEA) – Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products, EMEA 2006.
Title